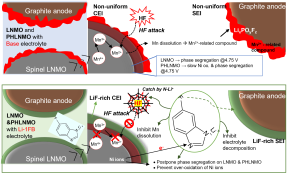
Spinel LiNi0.5Mn1.5O4 (LNMO) is one of the attractive cathodes due to several advantages such as Co-free for low cost and high operating voltage (∼5 V). Normally, there are Fd3m and P4332 space group types of LNMOs. However, both LNMOs suffer phase segregation at intragranular side which affects the lithium diffusion pathway and structural degradation during cycles. On the other hand, Jahn-Teller distortion on Mn3+ as well as HF effects from electrolyte oxidation at high voltage window triggered lead Mn dissolution from LNMO that causes performance fade. In this work, a new salt is developed, lithium 1-fluoro benzimidazole (Li-1FB) for LNMO with two different space groups. Ex-situ XRD results show the Li-1FB postpones the formation of intermediate phase transition on both LNMOs at different states of charge. In addition, 1H NMR analysis reveals the Li-1FB salt can prevent HF formation by the N-Li functional group of imidazole ring thus inhibits Mn dissolution on both LNMOs after cycling. Furthermore, the Li-1FB salt is suitable to improve cycle performance of both LNMOs in half-cell (Li metal as anode) and full cell (graphite as anode) applications. This new salt provides dramatic improvements, and it is suitable for further high-energy applications.
Investigation of space group effects of High-Voltage spinel LiNi0.5Mn1.5O4: Unveiling the influences of fluorinate benzimidazole salt additive
Investigations of an organic coverage to Ni-rich cathode materials: Effects on deteriorated, cathode electrolyte interphase, and chemical crossover
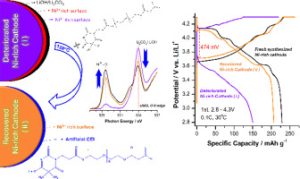 Ni-rich cathodes inherit surface residual lithium compounds (SRLCs) for several reasons, such as cation mixing, oxygen vacancies, and the spontaneous reduction of high-Ni valence ions. Consequently, Ni-rich compounds must be treated before use to maximize their performance. This study describes the development of an organic coverage (OC) that can be directly utilized with a deteriorated Ni-rich cathode. The OC is synthesized from 5,5-dimethylbarbituric acid and polyethylene glycol diacrylate to provide two functions for the deteriorated Ni-rich cathode surface: spontaneous ion exchange and the self-electrochemical oxidation of Ni ions. SRLCs, such as Li2CO3 and LiOH, decompose through a transformation reaction from the trioxo to the dioxo form of the OC structure. Then this lithiated OC forms an organic artificial cathode electrolyte interface on the cathode surface, which further reduces the effects of chemical crossover on the anode side. It is also believed that the dioxo form promotes Ni2+ self-oxidation on the surface of Ni-rich cathodes and recovers the original Ni3+ valence state by Li+ re-intercalation. Thus, the capacities of the deteriorated LiNiO2 and LiNi0.8Mn0.1Co0.1O2 recover almost to their original values and retain the same excellent cycle performance as that of the fresh compounds.
Ni-rich cathodes inherit surface residual lithium compounds (SRLCs) for several reasons, such as cation mixing, oxygen vacancies, and the spontaneous reduction of high-Ni valence ions. Consequently, Ni-rich compounds must be treated before use to maximize their performance. This study describes the development of an organic coverage (OC) that can be directly utilized with a deteriorated Ni-rich cathode. The OC is synthesized from 5,5-dimethylbarbituric acid and polyethylene glycol diacrylate to provide two functions for the deteriorated Ni-rich cathode surface: spontaneous ion exchange and the self-electrochemical oxidation of Ni ions. SRLCs, such as Li2CO3 and LiOH, decompose through a transformation reaction from the trioxo to the dioxo form of the OC structure. Then this lithiated OC forms an organic artificial cathode electrolyte interface on the cathode surface, which further reduces the effects of chemical crossover on the anode side. It is also believed that the dioxo form promotes Ni2+ self-oxidation on the surface of Ni-rich cathodes and recovers the original Ni3+ valence state by Li+ re-intercalation. Thus, the capacities of the deteriorated LiNiO2 and LiNi0.8Mn0.1Co0.1O2 recover almost to their original values and retain the same excellent cycle performance as that of the fresh compounds.
Auto-reduction of Au(III) on Fe-Doped, Pyrrolic-N-Modified Nanoporous Carbon Derived from ZIF-8 for the Electrochemical Immunosensing of CA-125
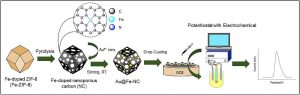 Ovarian cancer (OC) is associated with high late mortality owing to hidden symptoms in the early stages and limited screening measurements. Therefore, it is urgent to develop a highly efficient label-free immunosensor for targeting cancer antigens. In this study, we developed a gold-loaded iron-doped nanoporous carbon (denoted as Au@Fe-NC) as an ideal substrate for cancer antigen detection with high specificity and sensitivity. First, an Fe, N-doped nanoporous carbon (Fe-NC) material was synthesized by carbonizing a Fe-doped metal-organic framework at 900°C under continuous nitrogen flow. The resultant Fe-NC contains pyrrolic-N, which facilitates the auto-reduction of Au³⁺ to Au⁰ without requiring an external reducing agent. Thus, the Au@Fe-NC was spontaneously formed by mixing an aqueous HAuCl₄ solution with Fe-NC. The immunosensor developed using Au@Fe-NC exhibits outstanding stability as well as high sensitivity to carbohydrate antigen 125 (CA-125), a tumor marker, presenting a linear dynamic range of 0.1 pg mL⁻¹ to 1 µg mL⁻¹ and a detection limit of 0.1 pg mL⁻¹. The proposed Au@Fe-NC-based immunosensor has the ability to detect CA-125 in human serum samples and is a promising material for OC diagnosis.
Ovarian cancer (OC) is associated with high late mortality owing to hidden symptoms in the early stages and limited screening measurements. Therefore, it is urgent to develop a highly efficient label-free immunosensor for targeting cancer antigens. In this study, we developed a gold-loaded iron-doped nanoporous carbon (denoted as Au@Fe-NC) as an ideal substrate for cancer antigen detection with high specificity and sensitivity. First, an Fe, N-doped nanoporous carbon (Fe-NC) material was synthesized by carbonizing a Fe-doped metal-organic framework at 900°C under continuous nitrogen flow. The resultant Fe-NC contains pyrrolic-N, which facilitates the auto-reduction of Au³⁺ to Au⁰ without requiring an external reducing agent. Thus, the Au@Fe-NC was spontaneously formed by mixing an aqueous HAuCl₄ solution with Fe-NC. The immunosensor developed using Au@Fe-NC exhibits outstanding stability as well as high sensitivity to carbohydrate antigen 125 (CA-125), a tumor marker, presenting a linear dynamic range of 0.1 pg mL⁻¹ to 1 µg mL⁻¹ and a detection limit of 0.1 pg mL⁻¹. The proposed Au@Fe-NC-based immunosensor has the ability to detect CA-125 in human serum samples and is a promising material for OC diagnosis.
Evaluation of LiNiO2 with minimal cation mixing as a cathode for Li-ion batteries
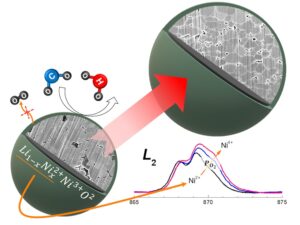 The electric vehicle (EV) market has grown tremendously in recent years, and is driving the development of lithium-ion batteries (LIBs) for energy storage. To meet the demand for high-energy–density and low-cost LIBs, Co-free and Ni-rich cathodes (e.g., LiNiO2; LNO) and Si anodes are being investigated. However, drawbacks of LNO, such as cation mixing, processing challenges, and safety risks significantly limit its commercialization. Increasing the oxygen partial pressure (𝑝𝑂2) during calcination of LNO has been shown to maintain its structural order and electrochemical performance. However, the mechanism by this observation is not well understood. In this research, three 𝑝𝑂2 conditions were applied during the calcination of LNO cathodes. The calcination 𝑝𝑂2 affects the sub-surface of LNO rather than the bulk region. Synchrotron spectroscopy and in-situ pressure analysis confirmed that Ni2+ and excess Li are present on the sub-surface of the LNO processed at the lowest 𝑝𝑂2. Increasing the 𝑝𝑂2 decreases the off-stoichiometry of LNO by providing additional oxygen to compensate for oxygen loss, especially at the sub-surface. A detailed mechanism by which the calcination 𝑝𝑂2 affects LNO is proposed. An LIB with the LNO cathode calcined at the highest 𝑝𝑂2 had a high initial capacity of 239.8 mA h g−1 (93.4 %), excellent fast charging cycle retention of half-cell and full cell at 55 °C, little gas evolution during cycling, and almost no weight change during storage in air. This calcining strategy prevents the cation mixing limitation of LNO, taking it one step closer to future EV application.
The electric vehicle (EV) market has grown tremendously in recent years, and is driving the development of lithium-ion batteries (LIBs) for energy storage. To meet the demand for high-energy–density and low-cost LIBs, Co-free and Ni-rich cathodes (e.g., LiNiO2; LNO) and Si anodes are being investigated. However, drawbacks of LNO, such as cation mixing, processing challenges, and safety risks significantly limit its commercialization. Increasing the oxygen partial pressure (𝑝𝑂2) during calcination of LNO has been shown to maintain its structural order and electrochemical performance. However, the mechanism by this observation is not well understood. In this research, three 𝑝𝑂2 conditions were applied during the calcination of LNO cathodes. The calcination 𝑝𝑂2 affects the sub-surface of LNO rather than the bulk region. Synchrotron spectroscopy and in-situ pressure analysis confirmed that Ni2+ and excess Li are present on the sub-surface of the LNO processed at the lowest 𝑝𝑂2. Increasing the 𝑝𝑂2 decreases the off-stoichiometry of LNO by providing additional oxygen to compensate for oxygen loss, especially at the sub-surface. A detailed mechanism by which the calcination 𝑝𝑂2 affects LNO is proposed. An LIB with the LNO cathode calcined at the highest 𝑝𝑂2 had a high initial capacity of 239.8 mA h g−1 (93.4 %), excellent fast charging cycle retention of half-cell and full cell at 55 °C, little gas evolution during cycling, and almost no weight change during storage in air. This calcining strategy prevents the cation mixing limitation of LNO, taking it one step closer to future EV application.
Organic redox flow battery: Are organic redox materials suited to aqueous solvents or organic solvents?
Redox flow battery (RFB) systems have been developed to meet both the high-capacity energy storage demands and the safety concerns associated with the commonly used lithium ion batteries (LIBs). After the successful commercialization of vanadium redox flow battery, it has been integrated into other redox systems, both organic and inorganic. The redox behaviour of organic molecules could be fine-tuned by functionalizing with suitable electron donating and withdrawing groups. Hence, organic redox materials became the prime focus for the electrochemical energy researchers and their sustainability in organic and aqueous solvents were systematically examined. This review focuses on the development made in the key aspects of solubility, redox potential, multi-electron redox centres, cross-over issue and stability, both in aqueous and organic mediums. Some of new interesting ideas such as single molecule redox targeting reactions, eutectic mixture, bipolar redox molecules and symmetric flow battery were also discussed. With the developments made, we present our viewpoint on which renewable energy storage technology is suitable for sustainable energy storage.
Failure Mechanisms of High-Voltage Spinel LiNi0.5Mn1.5O4 with Different Morphologies: Effect of Self-Regulation by Lithium Benzimidazole Salt Additive
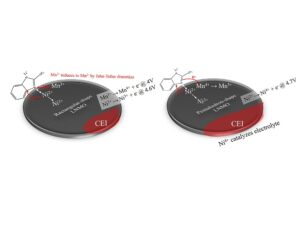
High voltage (∼5 V) spinel LiNi0.5Mn1.5O4 (LNMO) has attracted great attention because of its ultrahigh voltage plateau, which can be used as a cathode to reduce pressure in battery management systems. Moreover, compared with layered LiNxMyCzO2 materials, LNMO only requires little amounts of Ni, is cobalt-free for maintaining energy density, is inexpensive, and is lightweight. This study demonstrates two types of primary particles with different morphologies: rectangular and pentahedral. The pentahedron-shaped LNMO has lower surface energy owing to the formation of high valence Ni on the surface, thereby causing gas evolution and a loss in cycle retention, a direct Ni2+/Ni4+ reaction. Conversely, rectangular-shaped LNMO with higher Mn3+ content exhibits a stable electrochemical reaction, which provides a higher surface energy that prevents ethylene carbonate (EC) decomposition on the surface, and thereby, excellent performance is obtained, a parallel reaction of Mn3+/Mn4+ and Ni2+/Ni3+. By adding a lithium salt additive, trifluoromethyl benzimidazole (LiTFB), a self-regulation of Ni and Mn ion valences leads to a key reaction on both pentahedral (surface disordering effect) and rectangular (preventing Jahn–Teller distortion effect) LNMO morphologies. The two-electron transfer in the reactions of Ni2+/3+ and Mn3+/4+ of LiTFB-modified LNMOs provides excellent electrochemical performance for further high-energy applications.
In Situ Co–O Bond Reinforcement of the Artificial Cathode Electrolyte Interphase in Highly Delithiated LiCoO2 for High-Energy-Density Applications
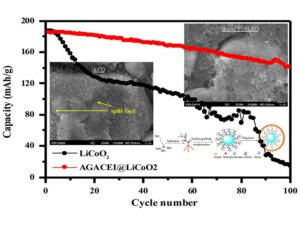 Highly delithiated LiCoO2 has high specific capacity (>200 mAh g–1); however, its degradation behavior causes it to have poor electrochemical performance and thermal instability. The degradation of highly delithiated LiCoO2 is mainly induced by oxygen vacancy migration and weakening of oxygen-related interactions, which result in pitting corrosion and fault formation on the surface. In this research, a coupling agent, namely, 3-aminopropyltriethoxysilane (APTES), was grafted onto the surface of LiCoO2 to form a cross-linking structure. Through the aza-Michael addition reaction, an oligomer formed from barbituric acid and bisphenol a diglycidyl ether diacrylate were reacted with the cross-linking APTES to form an artificial cathode electrolyte interphase (ACEI). The highly delithiated LiCoO2 containing the ACEI had considerably less degradation on the surface of the bulk material caused by oxygen release. The formation of the O1 phase was prevented in high delithiation and high-temperature operations. This research revealed that the ACEI reinforced the Co–O bond, which is crucial in preventing gas evolution and O1 phase formation. In addition, the ACEI prevents direct contact between the electrolyte and highly active surface of LiCoO2, thereby preventing the formation of a thick and high impedance traditional cathode electrolyte interphase. According to the present results, highly delithiated LiCoO2 containing the ACEI exhibited outstanding cycle retention and capacity at 55 °C as well as low heat capacity release in the fully delithiated state. The ACEI considerably protected and maintained the electrochemical performance of highly delithiated LiCoO2, which is suitable for high-energy-density applications, such as electric vehicles and power tools.
Highly delithiated LiCoO2 has high specific capacity (>200 mAh g–1); however, its degradation behavior causes it to have poor electrochemical performance and thermal instability. The degradation of highly delithiated LiCoO2 is mainly induced by oxygen vacancy migration and weakening of oxygen-related interactions, which result in pitting corrosion and fault formation on the surface. In this research, a coupling agent, namely, 3-aminopropyltriethoxysilane (APTES), was grafted onto the surface of LiCoO2 to form a cross-linking structure. Through the aza-Michael addition reaction, an oligomer formed from barbituric acid and bisphenol a diglycidyl ether diacrylate were reacted with the cross-linking APTES to form an artificial cathode electrolyte interphase (ACEI). The highly delithiated LiCoO2 containing the ACEI had considerably less degradation on the surface of the bulk material caused by oxygen release. The formation of the O1 phase was prevented in high delithiation and high-temperature operations. This research revealed that the ACEI reinforced the Co–O bond, which is crucial in preventing gas evolution and O1 phase formation. In addition, the ACEI prevents direct contact between the electrolyte and highly active surface of LiCoO2, thereby preventing the formation of a thick and high impedance traditional cathode electrolyte interphase. According to the present results, highly delithiated LiCoO2 containing the ACEI exhibited outstanding cycle retention and capacity at 55 °C as well as low heat capacity release in the fully delithiated state. The ACEI considerably protected and maintained the electrochemical performance of highly delithiated LiCoO2, which is suitable for high-energy-density applications, such as electric vehicles and power tools.
Controlling Ni2+ from the Surface to the Bulk by a New Cathode Electrolyte Interphase Formation on a Ni-Rich Layered Cathode in High-Safe and High-Energy-Density Lithium-Ion Batteries
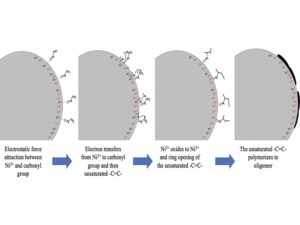
Ni-rich high-energy-density lithium-ion batteries pose great risks to safety due to internal short circuits and overcharging; they also have poor performance because of cation mixing and disordering problems. For Ni-rich layered cathodes, these factors cause gas evolution, the formation of side products, and life cycle decay. In this study, a new cathode electrolyte interphase (CEI) for Ni2+ self-oxidation is developed. By using a branched oligomer electrode additive, the new CEI is formed and prevents the reduction of Ni3+ to Ni2+ on the surface of Ni-rich layered cathode; this maintains the layered structure and the cation mixing during cycling. In addition, this new CEI ensures the stability of Ni4+ that is formed at 100% state of charge in the crystal lattice at high temperature (660 K); this prevents the rock-salt formation and the over-reduction of Ni4+ to Ni2+. These findings are obtained using in situ X-ray absorption spectroscopy, operando X-ray diffraction, operando gas chromatography–mass spectroscopy, and X-ray photoelectron spectroscopy. Transmission electron microscopy reveals that the new CEI has an elliptical shape on the material surface, which is approximately 100 nm in length and 50 nm in width and covers selected particle surfaces. After the new CEI was formed on the surface, the Ni2+ self-oxidation gradually affects from the surface to the bulk of the material. It found that the bond energy and bond length of the Ni–O are stabilized, which dramatically inhibit gas evolution. The new CEI is successfully applied in a Ni-rich layered compound, and the 18650- and the punch-type full cells are fabricated. The energy density of the designed cells is up to 300 Wh/kg. Internal short circuit and overcharging safety tests are passed when using the standard regulations of commercial evaluation. This new CEI technology is ready and planned for future applications in electric vehicle and energy storage.